HTML
Styling
Neurotransmitter
Pain Management
HTML Structure
Neurobiology
Pain
CSS
استكشاف دور الناقلات العصبية في الصداع
دور مركزي في علم الأمراض الصداع النصفي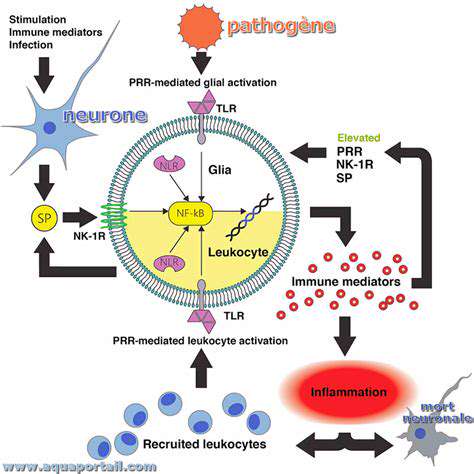
دور السيروتونين في نقل الألم
السيروتونين، وهو ناقل عصبي أساسي، يلعب دورًا معقدًا في مسارات إشارات الألم. فهو متورط في تعديل انتقال إشارات الألم من المحيط إلى النخاع الشوكي.
دور مادة P وبيبتيدات أخرى في آليات الصداع

Read more about استكشاف دور الناقلات العصبية في الصداع
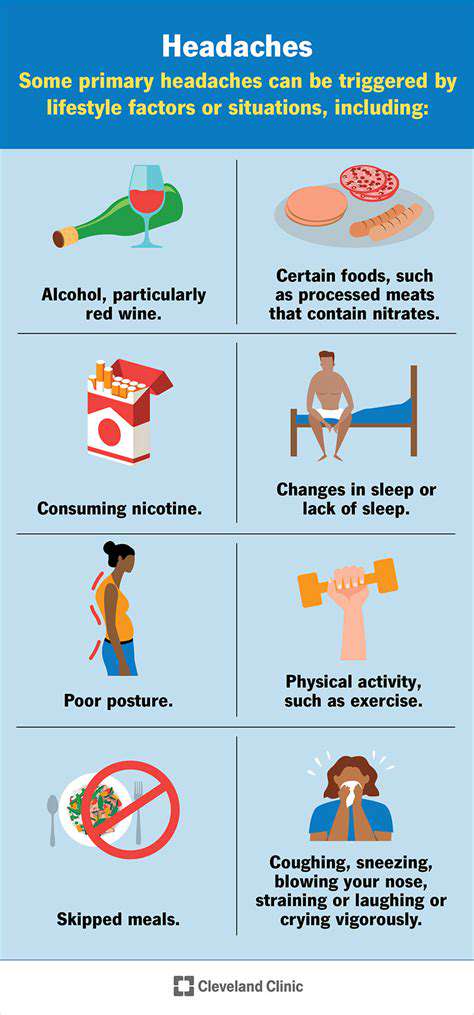
الأسباب والأعراض والعلاجات ومتى يجب طلب المساعدة. يمكن أن تنشأ آلام الرأس في الجانب الأيسر من حالات متنوعة ، بما في ذلك الصداع الناتج عن التوتر ، والشقيقة ، والصداع العنقودي. من الضروري التمييز بين هذه الأنواع من الألم لتحديد العلاج الفعال. الأسباب الشائعة - صداع التوتر: غالبًا ما يرتبط بالتوتر ، ويمكن أن تسبب هذه الصداع ألمًا باهتًا ومستمرًا. - الشقيقة: يتميز بألم شديد ونابض ، وعادة ما يصاحبه غثيان وحساسية للضوء. - الصداع العنقودي: شكل نادر ولكنه حاد من الصداع يحدث عادةً بنمط دوري. - العدوى الجيوبية واضطرابات TMJ: يمكن أن تسبب أيضًا آلامًا موضعية. الأعراض المصاحبة يمكن أن تختلف الأعراض ، لكنها غالبًا ما تشمل ألمًا حادًا أو نابضًا ، وغثيانًا ، وحساسية للضوء. يمكن أن يوفر تحديد الأعراض المصاحبة أدلة حيوية للتشخيص ، وتوثيق الأنماط يمكن أن يساعد المتخصصين في الرعاية الصحية. العلاجات المنزلية يمكن غالبًا العثور على الراحة من خلال العلاجات المنزلية مثل: - الكمادات الباردة أو الدافئة: فعالة في تخفيف التوتر. - الراحة في غرف مظلمة وهادئة: يساعد في تقليل الانزعاج. - الترطيب: أساسي للوقاية من الصداع المرتبط بالجفاف. - تقنيات الاسترخاء: يمكن أن تساعد تقنيات مثل التنفس العميق في تقليل مستويات التوتر. متى تطلب المساعدة الطبية من الضروري طلب المساعدة الطبية إذا كنت تعاني من ألم شديد ومفاجئ ، أو أي أعراض مقلقة مثل تغيرات في الرؤية أو ارتباك. إن الصداع المزمن الذي يؤثر على حياتك اليومية يتطلب أيضًا تقييمًا احترافيًا. للحصول على رؤى شاملة حول كيفية التعرف على الأعراض ، وتنفيذ العلاجات ، والتعرف على متى يجب البحث عن المساعدة المهنية ، استكشف دليلنا التفصيلي حول إدارة آلام الرأس في الجانب الأيسر.
Oct 10, 2024
وصف صفحة الويب لألم الرأس في الجانب الأيمن. استكشف الأسباب المتعددة والأعراض وخيارات العلاج لألم الرأس في الجانب الأيمن. افهم التشريح وراء الانزعاج، من الشقيقة وآلام الرأس الناتجة عن التوتر إلى مشاكل الجيوب الأنفية والصداع العنقودي. تعرّف على أدوية فعّالة من دون وصفة طبية، وتعديلات في نمط الحياة، وعلاجات بديلة يمكن أن تساعد في تخفيف الألم. يؤكد دليلنا على متى ينبغي طلب المساعدة الطبية، مما يضمن أن تكون على دراية وتمكين في إدارة صحتك. اكتشف رؤى قيمة تؤدي إلى تحسين الرفاهية وجودة الحياة. سواء كنت تبحث عن تخفيف فوري أو استراتيجيات طويلة الأجل، نقدم لك مصدرًا شاملاً لفهم ومعالجة ألم الرأس في الجانب الأيمن.
Oct 11, 2024
الأسباب، التأثير، واستراتيجيات التخفيفآلام الرأس والرقبة هي مشكلة شائعة تؤثر على كثير من الأفراد، وتؤثر بشكل كبير على حياتهم اليومية وإنتاجيتهم. تستكشف هذه الدليل الشامل الأسباب المختلفة، من الوضعية السيئة وتوتر العضلات إلى التوتر والحالات الطبية الكامنة. يناقش أهمية طلب المشورة الطبية المهنية عندما تستمر الألم، بالإضافة إلى العلاجات المنزلية الفعالة وتغييرات نمط الحياة التي يمكن أن تخفف الأعراض. تشمل الموضوعات الرئيسية ما يلي: - التأثير على الحياة اليومية: يمكن أن تعيق آلام الرأس والرقبة الأنشطة الروتينية وتخلق تأثيرات سلبية على الصحة النفسية. - الأسباب الشائعة: تعلم عن عوامل مثل توتر العضلات، التوتر والإصابات التي تساهم في الألم. - الاستشارة الطبية: فهم متى يجب طلب المساعدة المهنية وفوائد العلاجات المخصصة. - العلاجات المنزلية: استكشاف استراتيجيات فعالة مثل التعديلات الهندسية، والتمارين، وممارسات اليقظة. - العلاجات البديلة: اكتشف كيف يمكن أن تكمل الوخز بالإبر، والعلاج بالتدليك، والعلاج بتقويم العمود الفقري العلاجات التقليدية. بالنسبة لأولئك الذين يعانون من آلام الرأس والرقبة، فإن فهم هذه العناصر أمر حاسم لإدارة الألم بشكل فعال والرفاهية العامة. قد يؤدي إعطاء الأولوية لنهج شامل إلى تحسينات كبيرة في جودة الحياة.
Oct 15, 2024
فهم، أسباب وإدارة ما هو الألم النابض؟ الألم النابض هو شعور خفقاني إيقاعي يمكن أن يختلف في شدته وموقعه. غالباً ما يرتبط بحالات وعائية أو عصبية، فإن التعرف على خصائصه أمر ضروري للتشخيص والإدارة. تستكشف هذه المقالة الأسباب الشائعة، والعلاجات الفعالة، ومتى يجب طلب المساعدة الطبية لعلاج الألم النابض. الأسباب الشائعة يمكن أن ينجم الألم النابض عن مجموعة متنوعة من المشكلات، بما في ذلك: - المشكلات الوعائية: يمكن أن تؤدي حالات مثل ارتفاع ضغط الدم أو التشوهات الوعائية إلى خفقان ملحوظ. - المتلازمات العصبية: تلف الأعصاب الناتج عن حالات مثل اعتلال الأعصاب السكري. - العوامل العضلية الهيكلية: غالباً ما تنتج الصداع التوتري أو الصداع النصفي شعوراً بالخفقان. العلاجات الفعالة تشمل استراتيجيات إدارة الألم النابض: - أدوية غير وصوفية مثل الإيبوبروفين أو الباراستامول للحالات الخفيفة. - تعديلات نمط الحياة، بما في ذلك ممارسة الرياضة بانتظام وإدارة الإجهاد، لتخفيف الأعراض. - عند الضرورة، استشارة المتخصصين في الرعاية الصحية للعلاجات المخصصة. متى يجب طلب العناية الطبية من المهم طلب المساعدة الطبية إذا كان الألم النابض شديدًا، ومصحوبًا بأعراض مثل تغييرات في الرؤية، أو تورم، أو صعوبة في التنفس. يمكن أن تؤدي التدخلات المبكرة إلى نتائج صحية أفضل. التدابير الوقائية يمكن أن يسهم التوجه النشط الذي يتضمن ممارسة الرياضة بانتظام، والتغذية السليمة، وإدارة الضغط النفسي بشكل كبير في تقليل تكرار وشدة النوبات المستقبلية. لفهم شامل وإدارة فعالة للألم النابض، اقرأ المقال بالكامل.
Nov 09, 2024
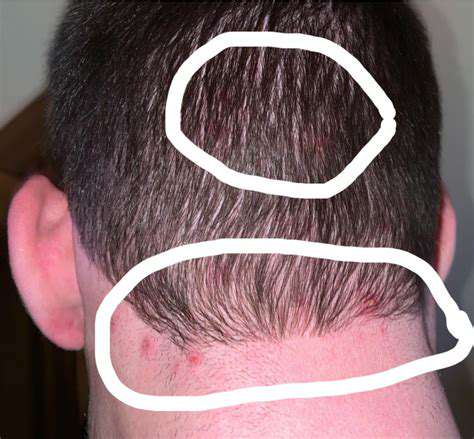
فهم الكتل في مؤخرة الرأساستكشف الأسباب الشائعة للكتل في مؤخرة الرأس، من الصدمات والإصابات إلى النمو الحميد مثل الخراجات والدهون. يستعرض هذا الدليل الشامل الأعراض المحتملة المرتبطة بهذه الكتل، ومتى يجب طلب المساعدة الطبية، وخيارات العلاج الفعالة. تعرف على العلاجات المنزلية لتخفيف الألم، والتدابير الوقائية لتجنب الإصابات المستقبلية، وأهمية فهم تشريح الرأس. سواء كانت كتلة صغيرة أو شيء أكثر خطورة، فإن معرفة الخطوات الصحيحة التي يجب اتخاذها أمر بالغ الأهمية لصحتك. قم بزيارتنا للحصول على رؤى يمكن أن تساعدك أنت أو أحد أفراد أسرتك على التعامل مع الكتل في الرأس بأمان وفعالية.
Nov 14, 2024
تخطي الوجبات وتقلبات السكر في الدم كمحفزات للصداع
May 03, 2025
كيف يمكن للتغيرات في الروتين أن تؤدي إلى الصداع
May 07, 2025
صداع يومي مستمر جديد (NDPH): ما يجب أن تعرفه
May 14, 2025
الفوائد النفسية لتتبع تحسينات الصداع النصفي
May 26, 2025