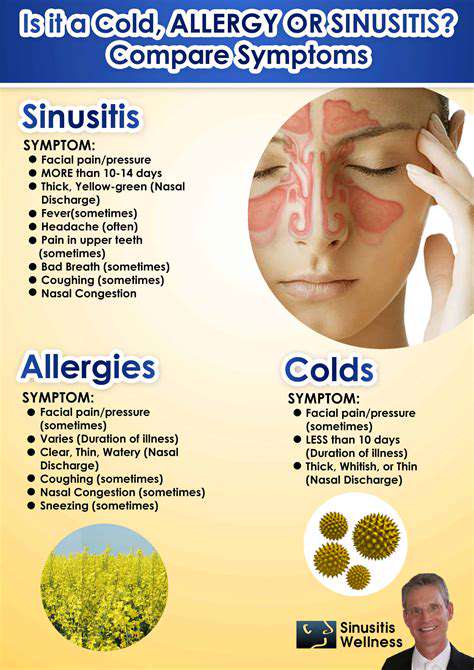
Neuromodulation Devices for Migraine Treatment (e.g., Cefaly, Nerivio)
Electrical stimulation is a common method employed by neuromodulation devices. This technique involves delivering controlled electrical impulses to specific nerve pathways. These impulses can interfere with the abnormal electrical activity that often underlies migraine attacks. The precise location and intensity of the stimulation are crucial, as they directly impact the effectiveness and safety of the treatment. This targeted stimulation aims to restore a more balanced neural environment, reducing the frequency and intensity of migraine attacks.
Transcutaneous Electrical Nerve Stimulation (TENS): A Non-Invasive Approach
One example of electrical stimulation is transcutaneous electrical nerve stimulation (TENS). TENS utilizes electrodes placed on the skin to deliver low-voltage electrical currents to the nerves. This non-invasive technique is often used to manage pain, including migraine pain. While TENS can provide temporary relief for some individuals, its effectiveness in treating chronic migraine requires further research and clinical trials.
Chemical Neuromodulation: A Different Approach
Beyond electrical stimulation, chemical neuromodulation offers another avenue for managing migraine. This method often involves delivering specific drugs or neurochemicals directly to the target area of the nervous system. This precise delivery system can help achieve higher concentrations of the therapeutic agent at the site of action, potentially maximizing therapeutic benefits and minimizing side effects. The development of targeted drug delivery systems is a key area of research in neuromodulation.
Targeting Specific Neural Pathways: Precision Engineering
Neuromodulation devices are designed to precisely target specific neural pathways implicated in migraine pathophysiology. This targeted approach ensures that the stimulation or chemical delivery is directed at the root of the problem, maximizing the effectiveness of treatment and minimizing potential side effects. By precisely identifying and addressing the neural circuits involved, these devices aim to disrupt the cascade of events that lead to a migraine attack.
Device Types and Their Applications
Various types of neuromodulation devices are currently being developed and tested for migraine treatment. Each device employs a unique approach to stimulation or chemical delivery, and its application depends on the specific needs and characteristics of the individual. Research continues to explore the optimal device types and parameters for specific migraine subtypes to enhance treatment outcomes.
Future Directions and Research
The field of neuromodulation for migraine treatment is constantly evolving, with ongoing research aiming to improve the efficacy and safety of these devices. Future advancements may involve more sophisticated targeting mechanisms, improved device designs, and personalized approaches to treatment. Further clinical trials and studies are essential to validate the long-term benefits and establish the optimal use of neuromodulation devices for migraine sufferers.
Cefaly: A Deep Dive into the Transcutaneous Device
Cefaly's Mechanism of Action
Cefaly, a transcutaneous neurostimulation device, operates on the principle of delivering precisely calibrated electrical pulses to specific cranial nerves. These targeted electrical impulses aim to interrupt the pain signals traveling along these nerves, thus reducing or eliminating the sensation of migraine pain. The device is designed to be worn comfortably on the forehead, allowing for continuous or intermittent stimulation, depending on the user's needs and the intensity of the migraine. This controlled stimulation can effectively modulate the brain's response to pain triggers, offering a potential non-invasive treatment option for migraine sufferers.
The specific mechanism by which Cefaly achieves its therapeutic effect is still under investigation, but researchers believe it may involve several factors. One potential mechanism is the modulation of pain pathways in the central nervous system. Furthermore, the device might influence the release of neurochemicals associated with pain perception. Cefaly's ability to target specific cranial nerves may also play a role in its effectiveness, as it allows for a more focused approach to pain management compared to some other headache treatments.
Cefaly: Clinical Studies and Efficacy
Clinical studies investigating the effectiveness of Cefaly have yielded promising results, suggesting potential benefits for migraine sufferers. The studies often involve controlled trials, comparing the outcomes of Cefaly use against placebo or standard migraine treatments. These studies have explored various parameters, including the reduction in migraine frequency, intensity, and duration. Data gathered from these trials generally demonstrates Cefaly's potential as a supplementary treatment option.
A significant portion of the research focuses on evaluating Cefaly's safety profile and long-term efficacy. These studies are crucial for determining the device's suitability for widespread use. Furthermore, researchers are investigating the optimal stimulation parameters and user experience to enhance the device's effectiveness and user compliance. Overall, the clinical evidence supporting Cefaly's efficacy continues to evolve, but current findings suggest a promising path forward for non-invasive migraine management.
While promising, it's important to note that individual responses to Cefaly can vary. Factors such as the type and severity of migraines, as well as individual patient characteristics, might influence the device's effectiveness. Further research is required to fully understand the factors that contribute to variability in treatment outcomes.
The ongoing research into Cefaly's clinical application is essential for refining its use and confirming its place within the spectrum of migraine treatments. Ultimately, a comprehensive understanding of its effectiveness and limitations will empower healthcare professionals to make informed recommendations for patients.
Further studies are also examining the long-term safety and tolerability of Cefaly, as well as its potential interactions with other medications. This comprehensive approach to research is essential for developing a comprehensive understanding of Cefaly's role in the management of migraine.
The long-term impact of Cefaly on migraine management is still being evaluated, but initial findings indicate a potential for significant improvements in patient well-being and quality of life.
Evaluating Efficacy and Safety of Neuromodulation Devices

Assessing Efficacy
Evaluating the efficacy of a treatment involves rigorously measuring its ability to produce the desired outcome. This often involves controlled studies, comparing the treatment group to a control group. Careful consideration must be given to the specific metrics used to assess success, ensuring they are relevant and measurable. A well-designed study is crucial to ensure that observed effects are truly attributable to the treatment and not due to other factors. This process requires statistical analysis to determine the significance and reliability of the results.
A key aspect of efficacy evaluation is the selection of appropriate outcome measures. These measures should be specific, sensitive, and reliable, accurately reflecting the impact of the treatment. For example, in a clinical trial evaluating a new drug for hypertension, blood pressure readings, adverse events, and patient reported outcomes would all be vital metrics. These data points, when analyzed collectively, provide a comprehensive understanding of the treatment's effectiveness.
Understanding Safety Profiles
Assessing the safety of a treatment is paramount. This involves identifying and characterizing any potential adverse effects, ranging from mild discomfort to severe complications. Detailed documentation of these effects is essential for understanding the potential risks associated with the treatment and for informing appropriate precautions.
Safety data is often collected through various methods, including monitoring for adverse events during treatment, conducting long-term follow-up studies, and analyzing laboratory results. This comprehensive approach allows for a thorough evaluation of the potential risks and benefits associated with the treatment.
Methodological Considerations
The methodology employed in evaluating efficacy and safety is critical. Rigorous study design, including randomization, blinding, and appropriate sample sizes, is essential to minimize bias and ensure the validity of the findings. The choice of appropriate statistical methods for analyzing the data is equally important. These methods should be suitable for the type of data collected and capable of accurately identifying significant trends.
Ethical considerations are paramount throughout the process. Informed consent from participants is essential, and the study must adhere to all relevant regulations and guidelines. Data integrity is paramount, and all procedures must be transparent and reproducible.
Interpretation and Reporting
Interpreting the results of efficacy and safety evaluations requires careful consideration of the context and limitations of the study. It's crucial to consider the potential biases present in the data and the generalizability of the findings to different populations. Proper interpretation requires a thorough understanding of the study design, data analysis methods, and the limitations inherent in the research. Disseminating the findings in a clear and transparent manner is equally important, allowing for critical appraisal by the scientific community.
Reporting findings should include a comprehensive description of the methodology, results, and limitations. This transparency facilitates peer review and allows for the replication and validation of the study. Clear and concise reporting promotes effective knowledge dissemination and facilitates the advancement of medical knowledge.